Mr Andrew Bowey, consultant orthopaedic spinal surgeon and Mr David Fender, consultant orthopaedic surgeon, have performed a world-first operation as part of an international clinical trial.
The operation was performed as part of the BRAIVE IDE study which will test the effectiveness of a new device to treat children with scoliosis. The trust also recruited the first patient to the trial.
Developed by medical device company Medtronic, the Braive growth modulation system uses a braid secured to the spine with screws to slow growth on the curved side of the spine, while allowing growth to continue on the other side.
About 4% of children globally have scoliosis, making it one of the most common paediatric orthopaedic deformities. It occurs when the vertebrae twist or rotate, causing the spine to curve into a “C” or “S” shape, rather than a straight line. It typically occurs in children and impacts girls more often than boys. Standard treatment options may include braces or spinal fusion surgery.
According to the National Scoliosis Foundation, an estimated 30,000 children a year receive a brace to treat their condition, while 38,000 patients are treated with spinal fusion. While successful in correcting the spine’s curve, spinal fusion causes vertebrae to fuse together into a single bone, which stops growth in that area of the spine
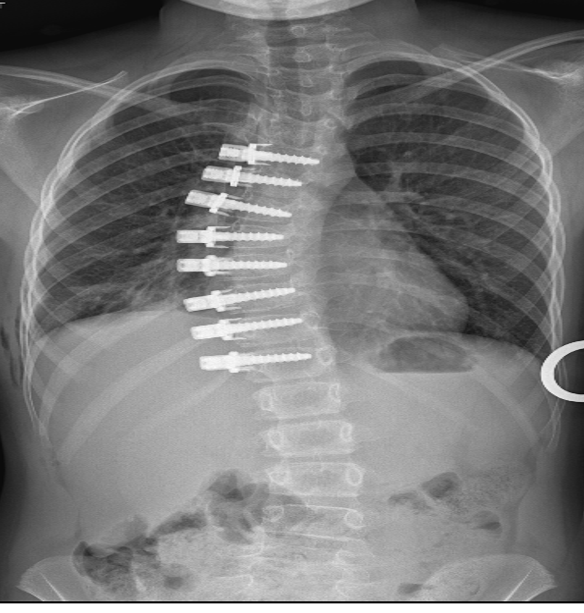
The BRAIVE IDE study will evaluate whether the system is safe and effective in correcting the spine’s curve in patients with juvenile or adolescent idiopathic scoliosis. The prospective, multi-centre study will enroll patients in the United States, Canada, and the United Kingdom.
Mr Bowey, who is also one of the study’s principal investigators, said:
“The Braive system is designed to correct scoliosis while allowing the spine to continue to grow, which is important for adolescents who are experiencing their most significant period of growth. I’m excited about its potential and the ability to bring this study to my patients who may benefit.”
Carlton Weatherby, vice president and general manager of Spine & Biologics within the Cranial & Spinal Technologies business, which is part of the Neuroscience Portfolio at Medtronic, added:
“Launching the BRAIVE IDE study is our latest step in bringing life-changing technologies to paediatric patients. As image guidance and navigation compatibilities extend further into additional spinal implant systems indicated for paediatric populations, they are coupled with a rapid cadence of transformative implant innovation.
“This uniquely positions us to offer the most comprehensive and integrated ecosystem of procedural solutions to paediatric spine surgeons driving meaningful improvements in clinical outcomes for young patients.”